












FDA (CDER) project billed as:
Make clinical observations queriable:
Metadata
Data (standards all the way down)
SELECT ?outcomeType ?dose
(AVG(?endpoint-time) AS ?rate)
WHERE {
# drug of interest
?adminDrug dt:CD.displayName "Upsidasium" ;
… codingSystem … .
# subjects in studies about drug
?arm :studySubject ?adminDrug ;
:studyParticipation ?subject .
# demographic selection
?subject :taxon ncbitax:9606 .
# outcomes assessing prescription performance
?outcome :intervention ?p ;
:value [ a ?outcomeType ] .
# ... of that drug on that subject (participation)
?p a :Prescription ;
:involvedSubject ?subject ;
:medication ?adminDrug .
} ORDER BY ?dose Establish common study data standards:
Success Criteria at end of the 5-year PDUFA V period:
code everything
minimize:
cope gracefully with:
interoperate with EHRS:
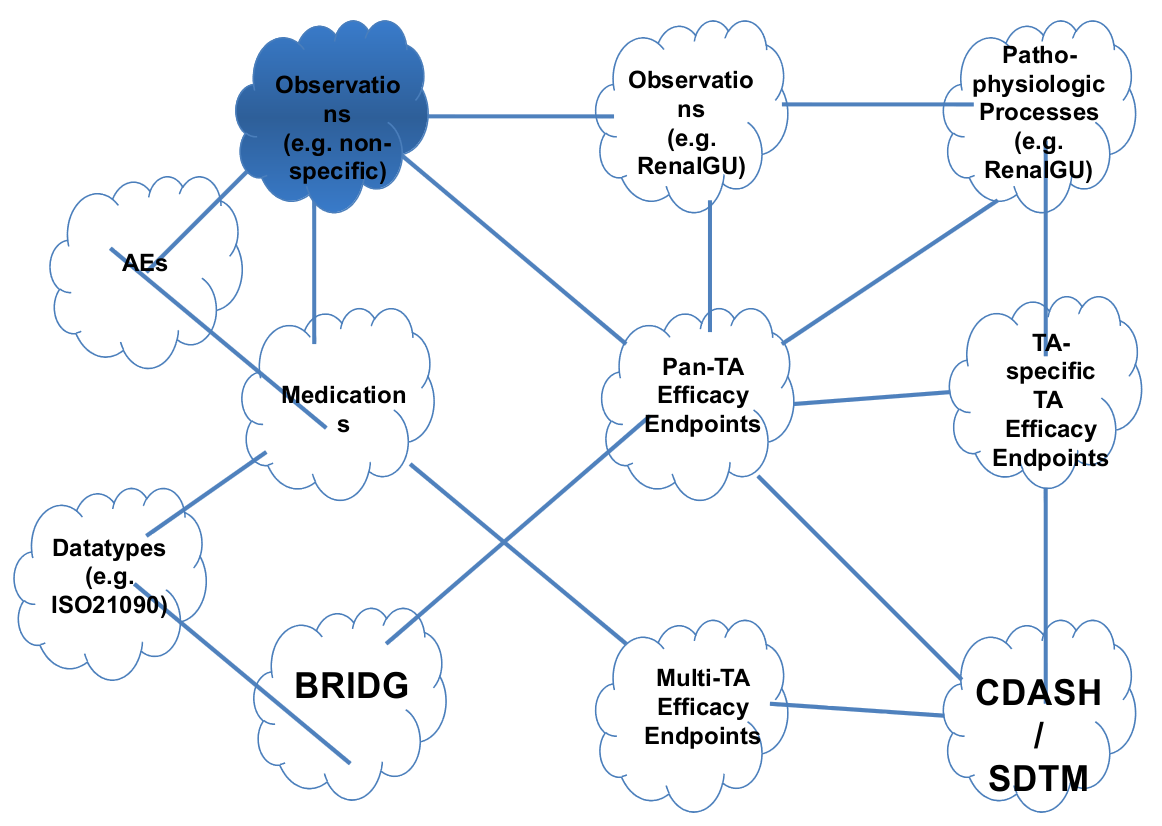
Existing Models/Standards
Controlled Terminologies:
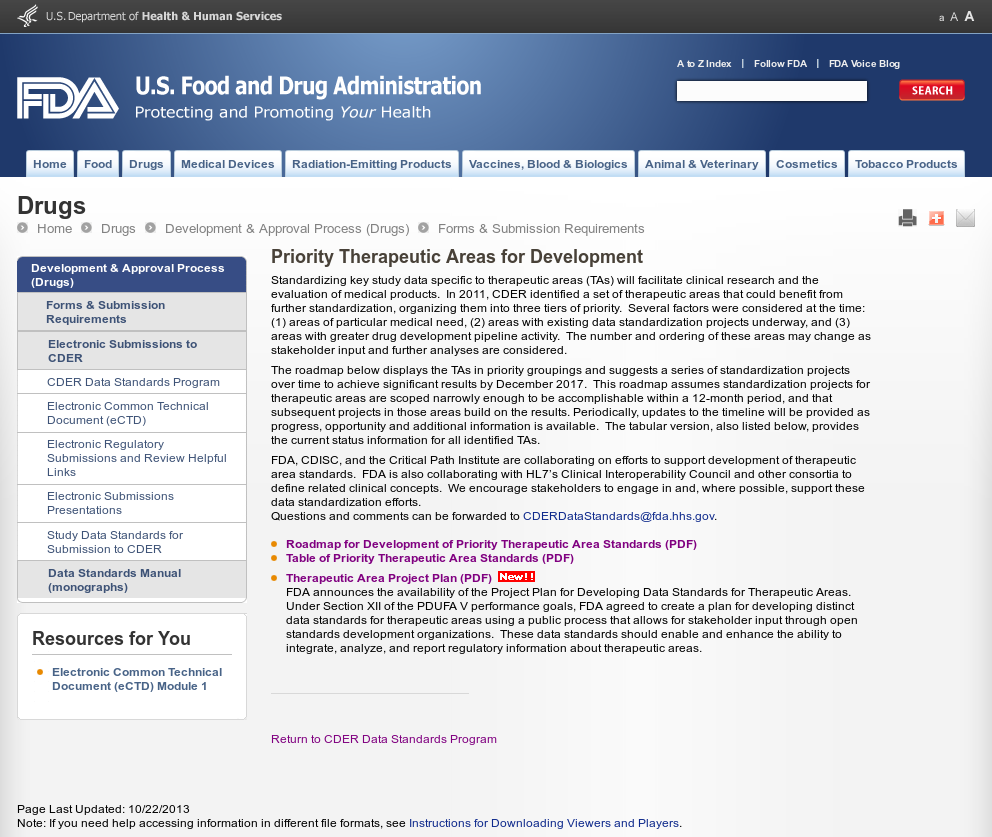
Therapeutic Efficacy is comprised of:
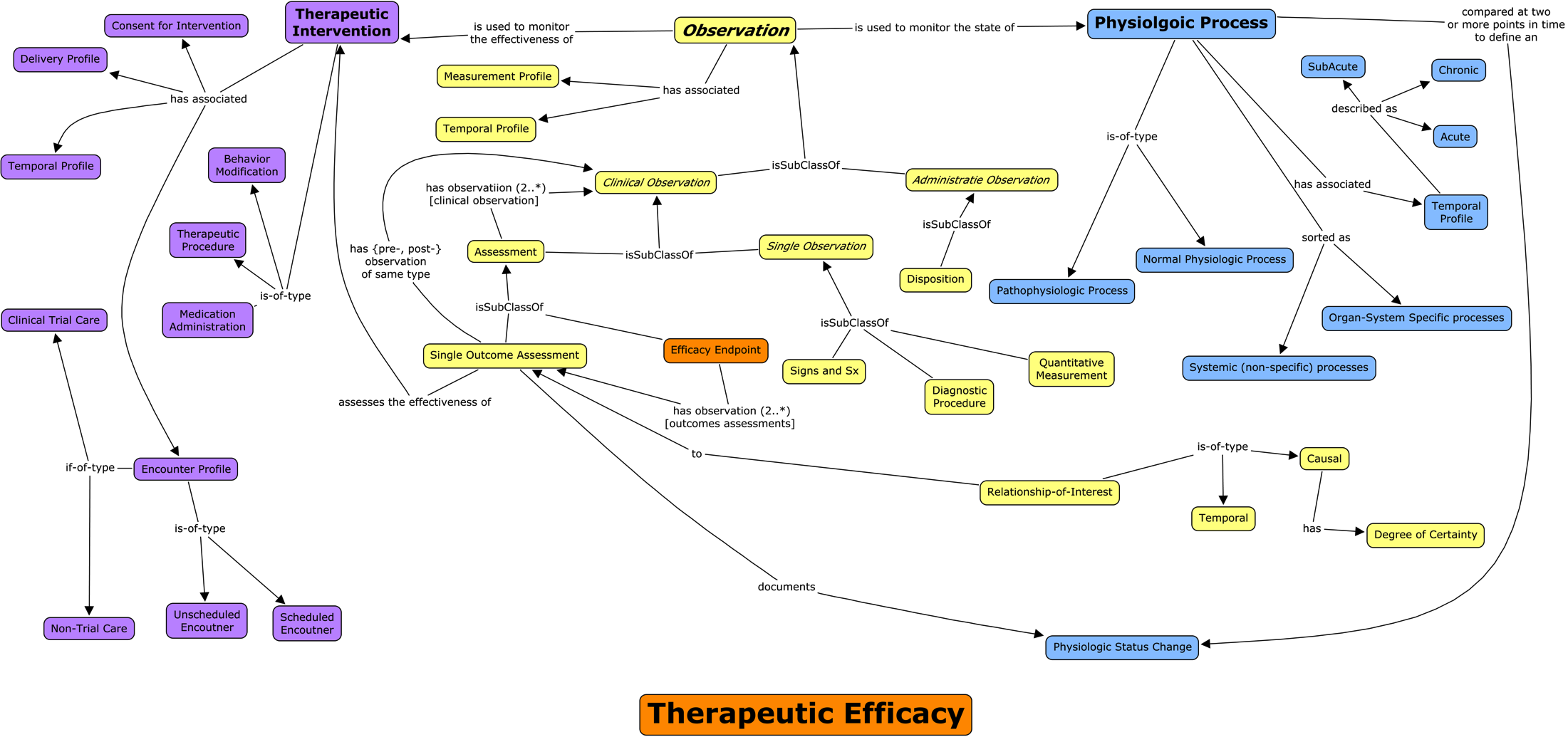

How is clinical data submitted today?
All lean on lists of study/domain-specific terms.
Issues:
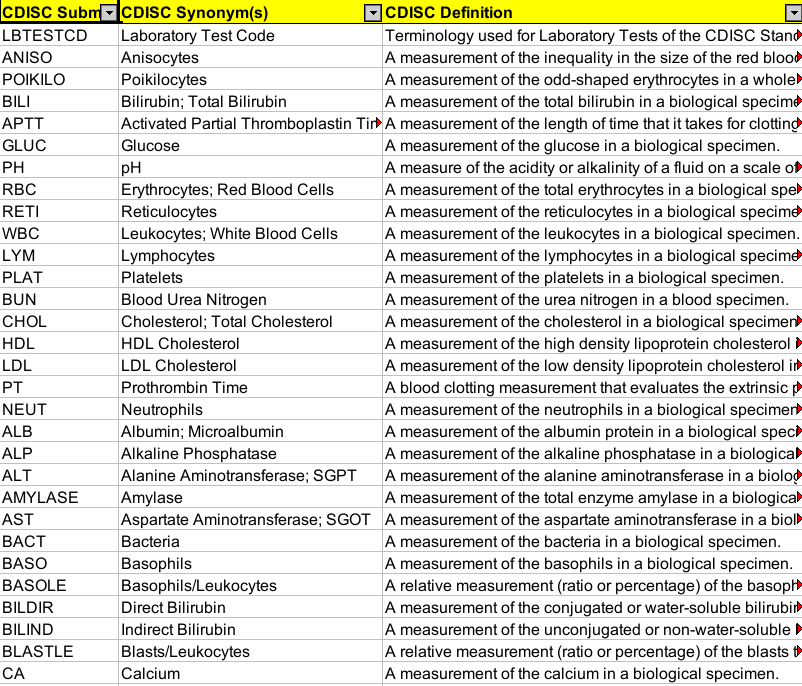
bridg:PerformedObservation
Portable BRIDG Representation
:SerumCreatinineLevel a owl:Class ; rdfs:subClassOf [ owl:onProperty bridg:resultIn ; owl:allValuesFrom bridg:PerformedClinicalResult ], [ owl:onProperty bridg:resultIn ; owl:cardinality 1 ] .
:GraftBPARAssessment a owl:Class ; rdfs:subClassOf core:NegativeOutcome ; owl:equivalentClass [ a owl:Class ; owl:intersectionOf ( [ a owl:Restriction ; owl:onProperty core:afterIntervention ; owl:someValuesFrom [ a owl:Restriction ; owl:onProperty core:hasPathologyFinding ; owl:hasValue :BanffIII ] ] [ a owl:Restriction ; owl:onProperty core:hasResultValue ; owl:hasValue :NonFunctioningGraft ] ) ] .
<http://www.w3.org/2013/12/FDA-TA/RenalTransplantation> a owl:Ontology ; owl:imports <http://www.w3.org/2013/12/FDA-TA/core> , <http://www.w3.org/2013/12/FDA-TA/renal> , <http://www.w3.org/2013/12/FDA-TA/transplant> .
Make clinical observations queriable:
?arm :studySubject ?adminDrug
SELECT ?outcomeType ?dose
(AVG(?endpoint-time) AS ?rate)
WHERE {
# drug of interest
?adminDrug dt:CD.displayName "Upsidasium" ;
… codingSystem … .
# subjects in studies about drug
?arm :studySubject ?adminDrug ;
:studyParticipation ?subject .
# demographic selection
?subject :taxon ncbitax:7609 .
# outcomes assessing prescription performance
?outcome :intervention ?p ;
:value [ a ?outcomeType ] .
# ... of that drug on that subject (participation)
?p a :Prescription ;
:involvedSubject ?subject ;
:medication ?adminDrug .
} ORDER BY ?dose
:subjectspostOpDay3GFR a rrej:RenalFunctionObservation ;
mm:observationTime "2013-07-08T14:50:00"^^xsd:dateTime ;
rrej:gfrFlowRate [ data:value 12.0 ; data:units ucum:mL-per-minute ].
:subjectsCSAR1 a rrej:RenalFunctionDiagnosis ; mm:observationTime "2013-07-08T14:52:00Z"^^xsd:dateTime ;
mm:isSupportedBy :subjectspostOpHour36GFR , :subjectsPostOpDay3UrineOutput , :subjectsPostOpDay3Temperature ,
:subjectsPostOpDay3SiteTenderness , :subjectsPostOpDay3SCr , :subjectspostOpDay3GFR .
:subjectsRenalBiopsy1 a rrej:RenalBiopsy ; mm:observationTime "2013-07-08T15:35:00Z"^^xsd:dateTime ;
mm:isJustifiedBy :subjectsCSAR1 ;
mm:labReport :subjectsRenalBiopsy1report .
:subjectsRenalBiopsy1report a rrej:RenalBiopsyReport ; mm:observationTime "2013-07-08T16:10:00Z"^^xsd:dateTime ;
mm:pathologyFinding rrej:BanffIII .
Would be in a table with codes in an EMR:
| obs id | when | coding system | ObsCode | value | units | addressing…performer… |
|---|---|---|---|---|---|---|
| 1234 | 2013-07-08T14:50:00 | CPT | 82565 | 12 | mL-per-minute | |
| 5678 | 2013-07-08T14:50:00 | SNOMED 2014-01-01 | 241374009 | 12 | mL-per-minute | |
| 4321 | 2013-07-08T14:50:00 | LOINC | 48643-1 | 12 | mL-per-minute |
or C-CDA:
<observation classCode="OBS" moodCode="EVN"> <code code="48643-1" codeSystem="2.16.840.1.113883.6.1" codeSystemName="LOINC" displayName="Glomerular filtration rate/1.73 sq M.predicted.black" /> <effectiveTime value="201307081450"/> <value type="PQ" value="12.0" unit="mL/min"/> <interpretationCode/> <referenceRange /> </observation>
_:obsX bridg:PerformedObservationCode [
hl7:coding [ dt:CDCoding.code "48643-1" ;
dt:CDCoding.codeSystem "2.16.840.1.113883.6.1" ;
dt:CDCoding.codeSystemName "LOINC" ;
dt:CDCoding.displayName "Glomerular filtration rate/1.73 sq M.predicted.black" ]
].
vs.
_:obsX bridg:PerformedObservationCode loinc:48643-1 .
A "subject" is a subject participation.
Activities come in four flavors e.g.
How do I link a bridg:Drug to an bridg:PerformedSubstanceAdministration?


:RenalFunctionObservation rdfs:subClassOf mm:QuantitativeMeasurement .
:RenalBiopsy
rdfs:subClassOf
mm:DiagnosticProcedure ,
[ owl:onProperty mm:isJustifiedBy ; owl:minCardinality 1 ] ,
[ owl:onProperty mm:labReport ; owl:cardinality 1 ] .
:RenalBiopsyReport
rdfs:subClassOf
mm:PathologyReport ,
[ a owl:Restriction ;
owl:onProperty :pathologyFinding ;
owl:someValuesFrom [ owl:oneOf ( :banfI :banfII :banfIII ) ] ] .
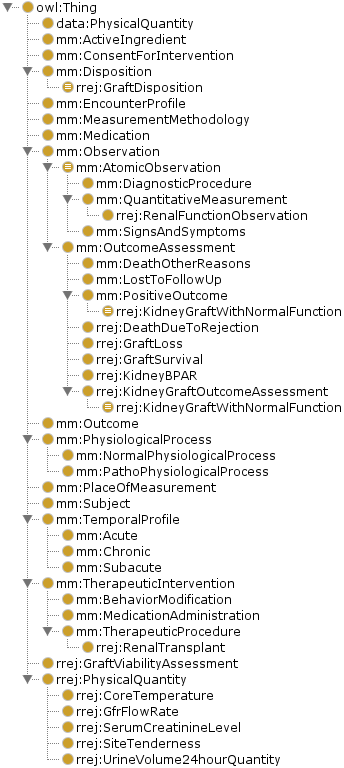
These don't capture: